Pharmacokinetics of Cefpodoxime Proxetil with Special Reference to Biochemical Parameters, Tissue residue and Spermatozoa motility in rats
DOI:
https://doi.org/10.5530/jcrsci.2.6Keywords:
Kinetics, Cefpodoxime, Tissue residueAbstract
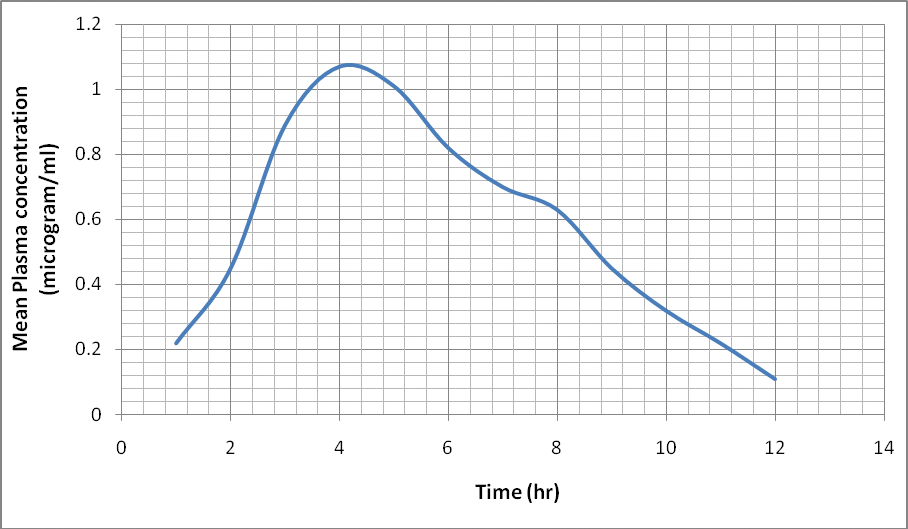
Studies on oral kinetics (blood and tissues) after single therapeutic dose of cefpodoxime (20 mg/kg oral) in rats of either sex and on some biochemical parameters, tissue residue and spermatozoa motility in male rats after cefpodoxime administration (20 mg/kg oral bid 7days) were undertaken so that generated data could be extrapolated to humans. For kinetic studies, 24 Wister rats of either sex, 3 months of age, (180-210 gm) were used. (Group I-IV; n=6) Blood samples collected from each animal of Group IV through heart puncture at 0 hour to serve as predrug control. All the group (I-IV) received cefpodoxime proxetil 20 mg/kg once orally as a single dose. At the end of 1, 4, 12 and 24 hour post oral administration, Group I, II, III and IV were utilized for kinetic studies. Blood samples were collected from each animal and vital organs viz brain, lung, liver, spleen, kidney and heart were dissected out for drug analysis and determination of weight. For bio- chemical parameters, tissue residue and spermatozoa motility, twelve male rats were randomly divided into Groups A and B (n=6) Group B received cefpodoxime (20 mg/kg orally bid 7 days) while Group A served as control. Biochemical parameters [Blood glucose, protein, Aspartate transaminase (AST), Alanine transaminase (ALT) and hemoglobin] were measured at 0 and 7th day while sperm count (total, live and dead) and mean organ weight (study and control group) and tissue residue of drug were evaluated at the end of treatment. Absorption of cefpodoxime was observed at 2 hour and reached a maximum at 4 hour and persisted in blood till 24 hour. Elimination half life in lung was highest followed by heart, liver, kidney and spleen while t½ k in plasma was very low suggesting more affinity of cefpodoxime for tissues than blood. Blood glucose, protein, AST and ALT activities were not significantly altered but the hemoglobin level and total and live sperm count decreased significantly in the study group compared to the control group. Residual level of cefpodoxime was highest in liver followed by kidney and other study organs. Therefore, the drug should be used in human beings judiciously and further study on human subjects is warranted.
Downloads




Quick Links